Nickel
hydroxide is commonly used as an active material for nickel
positiveelectrodes in Ni-based rechargeable batteries, electrochromic
devices, and as promising catalysts for oxygen evolution
reaction (OER). Some properties of nickel hydroxide electrodes
(such as high power density, very good cyclability, and
high specific energy) have made them very viablefor an extended
range of applications. It is reported that there are four
phases produced over the lifetime of a nickel hydroxide
electrode, namely, a-Ni(OH)2,
g-NiOOH, b-NiOOH, and b-Ni(OH)2 and the electrochemical
reactions of nickel hydroxides can be shown 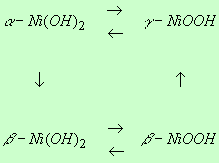 simply
by:
simply
by:
In
a battery application a « g cycling has a couple of advantages
over b « b cycling. First, more electrons are exchanged
per nickel atom in the a « g cycling because the oxidation
state of nickel in g-NiOOH is higher than that of b-NiOOH.
The
formation of g-NiOOH is associated with the volume expansion
or swelling of the nickel hydroxide electrode. The phase
change from b-NiOOH to g-NiOOH can be correlated to a 44
% increase in volume. It was reported that a stabilized
a-Ni(OH)2 electrode has a much larger charge
capacity than a b-Ni(OH)2 electrode.
Second, on prolonged charging, b-NiOOH is known to be converted
to g-NiOOH, causing a mechanical deformation which results
in an irreversible damage to the electrode. On the other
hand, a-Ni(OH)2 can be cycled to g-NiOOH reversibly
without any mechanical deformation. Therefore, a-Ni(OH)2
is expected to be a better electroactive material for a
high performance nickel positive electrode.
The
presence of certain metal cations in hydrated nickel oxides
and oxyhydroxides, generically referred as nickel hydrous
oxides, can strongly affect the electrochemical characteristics
of this interesting electrode material. Cobalt, cadmium
and zinc are commonly used as beneficial additives in nickel
battery electrodes.
The addition of several percent of additives, such as Cd,
Co, and Zn, to the nickel hydroxide is a very effective
method of suppressing the formation of g-NiOOH. Although
there are numerous studies on these topics, the results
are still not complete. In our laboratory the research about
the influence on different additives on the optical and
electrochemical response is currently under study.