|
Conducting
Polymers As Model-Membranes For Electrochemical Drug Release
Systems
The
development of pharmacological drugs had eveolved from the
botanic period, in the beginning of human civilization,
to the synthetic chemistry era, in the middle of the XXth
century. In the same way, new drugs appaered with very specific
actions and being able to control and to prevent a variety
of deceases. The administration and dosage of these phamacological
drugs had also evolved inthe last 100 years from conventional
pills to controlled/sustained relase systems with programable
rate.
Two
very important factors justify the search of new drug relase
systems: (i) The oscillation of the “in vivo”drug
concentrations higher and lowr to the minimum level and
(ii) the necessity of systems (i.e adhesives or implants)
that make possible and efficient the administration of “drug
cocktails” that, in the present form, they demant patients
to take up to 40 pills a day.
he
term “controlled release”implies in the prediction
and reproductibility of the drug release kinetics and the
wide variety of controlled relase systems can be classified
depending on the release technique used. The following table
shows some examples some examples where polymers are used
in these systems, calling attention to the technique under
study in our laboratory.
Table 1:
Some mechanisms producing drug release in the human organism.
| Release
agent |
Polymer |
Mechanism |
| pH |
Acid
or basic hydrogel |
pH
changes- swelling-drug release |
| Ionic
Force |
Ionic
hydrogel |
Changes
in the ionic force— changes in the hydrogel effective
charge— conformational changes – drug release. |
| Chemical
species |
Hydrogel
containing electron acceptor groups |
Charge
transfer complex formation- conformation change- drug
release |
| Enzyme
-substrate |
Hydrogel
containing immo-bilized enzymes |
Substrate
– enzymatic convertion – product produces
the conformational change of the hydrogel – drug
release |
| Magnetic |
Magnetic
particles dispersed in microespheres |
Magnetic
field applied- changes in the microsphere pores –
drug release |
| Sonication |
poly(etilene-vinil
alcool) |
Sonication-
temperature rise –drug release. |
| Electric |
Polyelectrolite |
Electric
field – charging of the polyemric membrane –
change of the electric charge of the polymer –
drug release |
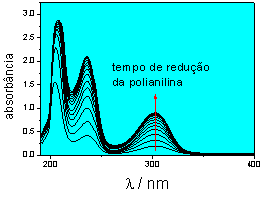 Fig.1.
Characteristic salicilic acid UV-Vis spectra, obtained in
a solution where the electrorelease is performed Fig.1.
Characteristic salicilic acid UV-Vis spectra, obtained in
a solution where the electrorelease is performed
Polyaniline
membranes capables to store both anionic or cationic drugs
and being also able to release them by the application of
potential pulses, are being developed in our laboratory.
We have already verified the capability and good performace
of this system in relaseing a model drug as salycilic acid
by the electrochemical reduction of a doped polyaniline
membrane (Fig. 1). By modifying the polyaniline membrane
with Nafionâ, the libearation of a model cationic
drug as dopamine is possible by oxidating the system.
Drug
release from the polymeric system to an aqueous enviroment
is followed by UV-Vis spectroscopy and form these data it
is possible to plot drug release curves (Fig 2).

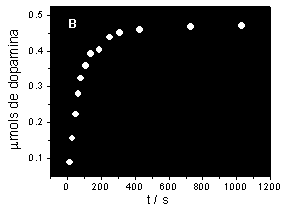
Fig.2.
Drug release curves. A: Salycilic acid from a polyaniline
membrane. B: Dopamine from the polyaniline/NáfionÒ
system.
|